News Release
<< Back
Introducing RediTrex®, A New Methotrexate Delivery System
"The RediTrex product line offers patients an important new choice in convenience, safety and dosing accuracy for their methotrexate therapy," said
RediTrex is FDA approved for patients with severe, active rheumatoid arthritis and polyarticular juvenile idiopathic arthritis who have difficulty tolerating or responding to orally delivered methotrexate. It is also approved for symptomatic control of severe, recalcitrant, disabling psoriasis in adults who are not adequately responsive to other forms of therapy.
Arthritis is the most common cause of disability in the
Current injectable MTX options available may not optimally meet the needs of an arthritis patient. Patients are offered either a vial and syringe for self-injection or the use of an expensive autoinjector. The vial and syringe method can be difficult for a patient to handle due to limited dexterity in their hands. In addition, obtaining the exact dose needed while preventing skin exposure to the caustic MTX can be quite challenging. The autoinjectors provide a better alternative to the vial and syringe but they remove injection control from the patient and can be painful to administer. They also resemble biologic autoinjectors, potentially causing confusion, and are more costly.
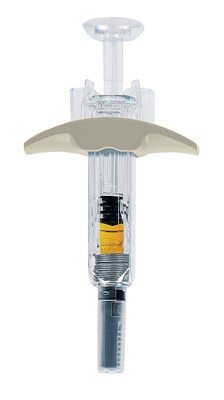
RediTrex has been designed to be the injectable MTX delivery system that optimally meets the needs of rheumatoid arthritis patients. RediTrex pre-filled syringes assure accurate safe dosing with an automatic retractable needle to reduce the risk of needle sticks. Each syringe is designed for ease of handling and has a large grip and concave plunger that allows patients with limited dexterity to self-administer at a controlled speed. The presentation also limits the possible confusion with biologic pens. In addition, RediTrex uses an extra-thin 29 gauge needle to reduce the pain of each administration. Lastly, RediTrex provides these benefits while being less expensive than autoinjectors.
RediTrex will be launched in two phases. Initial activity will focus on a limited number of centers and physicians as the organization gains insight into information needs and required patient support. During the first quarter of 2021, the second phase will expand activities to a full national launch to the Rheumatology market.
For more information, including full prescribing and safety data, please see www.reditrex.com
- Arthritis Prevalence and Statistics www.verywell.com/arthrities-prevalaence-and-statistics-189356
- Yazici Y, Bata Y. Parenteral methotrexate for the treatment of rheumatoid arthritis. Bulletin of the
Hospital for Joint Diseases - 2013. Lee J. Pelkey P , et al. Am Health Drug Benefits 2017; 10(1):42-48
About
- Acetadote® (acetylcysteine) Injection, for the treatment of acetaminophen poisoning;
- Caldolor® (ibuprofen) Injection, for the treatment of pain and fever;
- Kristalose® (lactulose) for Oral Solution, a prescription laxative, for the treatment of chronic and acute constipation;
- Omeclamox®-Pak, (omeprazole, clarithromycin, amoxicillin) for the treatment of Helicobacter pylori (H. pylori) infection and related duodenal ulcer disease;
- Vaprisol® (conivaptan) Injection, to raise serum sodium levels in hospitalized patients with euvolemic and hypervolemic hyponatremia;
- Vibativ® (telavancin) Injection, for the treatment of certain serious bacterial infections including hospital-acquired and ventilator-associated bacterial pneumonia, as well as complicated skin and skin structure infections;
- RediTrex® (methotrexate) Injection, for the treatment of active rheumatoid, juvenile idiopathic and severe psoriatic arthritis, as well as disabling psoriasis.
For more information on Cumberland's approved products, including full prescribing information, please visit the individual product websites, links to which can be found on the Company's website: www.cumberlandpharma.com.
The Company also has a series of Phase II clinical programs underway evaluating its ifetroban product candidates in patients with cardiomyopathy associated with Duchenne Muscular Dystrophy ("DMD"), Systemic Sclerosis ("SSc") and Aspirin-Exacerbated Respiratory Disease ("AERD").
Forward-Looking Statements
This press release contains forward-looking statements, which are subject to certain risks and reflect Cumberland's current views on future events based on what it believes are reasonable assumptions. No assurance can be given that these events will occur. As with any business, all phases of Cumberland's operations are subject to factors outside of its control, and any one or combination of these factors could materially affect Cumberland's results of operations. These factors include market conditions, competition, an inability of manufacturers to produce Cumberland's products on a timely basis or failure of manufacturers to comply with regulations applicable to pharmaceutical manufacturers, maintaining an effective sales and marketing infrastructure, natural disasters, public health epidemics, and other events beyond our control, as more fully discussed in the Company's most recent Form 10-K and subsequent 10-Qs as filed with the
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/introducing-reditrex-a-new-methotrexate-delivery-system-301175054.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/introducing-reditrex-a-new-methotrexate-delivery-system-301175054.html
SOURCE
Investor Contact: Erin Gull, Corporate Relations, (615) 255-0068; Media Contacts: Jeff Bradford, Bradford Dalton Group, (615) 515-4880 office, (615) 337-0964 mobile or Molly Aggas, Bradford Dalton Group, (704) 641-6641 mobile